The Use of Formaldehyde in Fumigation
Formaldehyde is a simple chemical compound made of hydrogen, oxygen and carbon. HCHO.
All life forms – bacteria, plants, fish, animals and humans – naturally produce formaldehyde as part of cell metabolism. Formaldehyde is also produced in the human body as a part of normal functions to build the basic materials needed for important life processes. Studies show that formaldehyde does not accumulate in people or animals because it is quickly broken down by the body’s natural metabolic processes. In the environment, formaldehyde is quickly broken down in the air by moisture and sunlight, or by bacteria in soil or water. Uses of formaldehyde are effectively regulated, and government oversight has been extensive. The U.S. Food and Drug Administration (FDA) has reviewed the safety of formaldehyde and approved its use as an indirect food additive in a number of materials having contact with food. The U.S. Department of Labor’s Occupational Health and Safety Administration (OSHA) has standards for workplace exposures to formaldehyde that provide comprehensive protection for employees through the implementation of good industrial hygiene practices.
Facts about Formaldehyde:
- Formaldehyde is a simple chemical compound made of hydrogen, oxygen and carbon. Molecular Formula: CH2O or HO(CH2 O)xH or H2CO or HCHO
- Formaldehyde is a colorless poisonous gas synthesized by the oxidation of methanol and used as an antiseptic, disinfectant, histologic fixative, and general-purpose chemical reagent for laboratory applications.
- Formaldehyde is readily soluble in water and is distributed as a 37 to 50, most commercially (37-40) % solution in water formalin. When formalin is heated formaldehyde vapor is generated.
- Formaldehyde is a flammable gas with a strong smell. It cause Irritation of the skin, eyes, nose, mucous membranes and throat occurred in some individuals with short-term exposure to concentrations of formaldehyde in air. It must be handled with caution when used as a disinfectant.
Risk of hatching egg contamination :
Good cleaning and disinfection practices are widely acknowledged as part of the optimisation of the viable day old chick (DOC) production in a hatchery. Good egg hygiene has often proven to improve the viability and quality of the DOC. The effect of bacterial penetration through the eggshell will affect hatching quality, such as early embryonic mortality, egg yolk infection, DOC mortality before hatching, besides significantly higher mortality and heterogeneity of the chicks during the first week of age. Obviously , various micro-organisms can contaminate the hatching eggs through vertical or/ and horizontal contamination(i.e Salmonella spp., Mycoplasma spp. E.coli, Pseudomonas, Staphylococcus, Proteus spp. and Aspergillus fumigatus).
A major source of contamination within the hatchery is the poor sanitary condition of the hatching eggs on arrival at the hatchery. The level of cleanliness of the hatchery, therefore, depends to a large extent on the hygienic standards of the laying flocks and, in particular, on the regular and frequent collection of eggs, egg truck transportation from farm to the hatchery , clean of feed ,water, egg collection equipments inside the house-farm, litters condetions, feed, water, dust, air circulations, employees, insects, mouses etc. In each hatchery, it should be mandatory that only clean eggs be set. These eggs should be kept clean out of contaminations sources on the farm, as soon as possible after collection to enable the destruction of microorganisms before these have time to penetrate through the eggshell.
Hygiene measures during the handling of eggs and day-old chicks(source: OIE / WHO):
- Egg handlers in the hatchery should wash their hands with soap and water and change to clean outer garments before handling hatching eggs received from the poultry farm.
- Chick sexers and chick handlers must wash and disinfect their hands and change into clean protective clothing and boots before commencing work and between different lots of chicks.
- Day old chicks or other poultry must be delivered or distributed in new chick boxes; or in used boxes made of suitable material which have been thoroughly cleaned and disinfected or fumigated.
- The chicks should be delivered directly from the hatchery by personnel wearing clean, disinfected outer clothing. Outer clothing should be changed or disinfected between each delivery.
- The delivery truck must be cleaned and disinfected before loading each consignment of chicks.
Recommendations applicable to hatching egg hygiene and transport(source: OIE / WHO):
- The litter in the laying house should be kept dry and in good condition. The nest box litter should be clean and adequate in quantity.
- Eggs should be collected at frequent intervals of not less than twice per day and placed in clean disinfected containers.
- Dirty, broken, cracked, leaking and dented eggs should be collected in a separate container and should not be used for hatching purposes.
- The clean eggs should be sanitised as soon as possible after collection.
- The sanitised eggs should be stored in a clean, dust free room used exclusively for this purpose and kept at low temperature less than 20°C and at a relative humidity of 70-80%.
- The eggs should be transported to the hatchery in new or clean cases which have been fumigated or sanitised with a liquid disinfectant. The cleaning and disinfection of vehicles must be a regular part of the hatchery routine.
Egg fumigation using formaldehyde:
Formaldehyde widely used in hatcheries as the most effective disinfectant that prevents and kill most of the microorganisms coming with the hatching eggs or other objects, although formaldehyde banned from use in some countries, and some of the companies looking for alternative disinfection instead of use formaldehyde, but can’t provide this goal up to now due to the power of formaldehyde to kill the pathogens with financially low cost.
The requirements to maximize germicidal activity from formaldehyde: 1) Temperature: the temperature range of 21-25°C. 2) Humidity: 60-80 %. 3) Time of exposure: the time required to kill the microorganisms depends on the temperature, the humidity and the concentration of formaldehyde, mostly take 20 minutes. 4) The concentration of formaldehyde, size of fumigation chamber or hall by cubic mater (WxLxH) 5) circulation of gas: how to distribute gas eventually around eggshell.6) the amount of organic matter on the shell surface. 7) Method used of generating formaldehyde vapours: Three basic methods that use liquid formalin alone, adding potassium permanganate to liquid formalin and paraformaldehyde powder, as follow:
- The volatilisation of the polymer paraformaldehyde under controlled temperature. At average 60 C; Heating 91 percent paraformaldehyde at the rate of 5-10 grams per cubic metre of space in an electric evaporator . From 10 to 20 minutes.
- Another common way used in the poultry industry was the addition of liquied formalin to potassium per- manganate (KMnO4) in 2:1 ratio (v/w). Adding 35 mL of formalin (40 percent formaldehyde) to 17.5 g potassium permanganate “KMNO4” per one cubic metre of space for 20 Minutes. When mixing with potassium permanganate for fumigation, always add the formalin to the potassium permanganate, never the reverse. Formaldehyde at bactericidal concentrations is very irritating to the eyes, nose and throat. Hatchery personnel should use a respirator and avoid unnecessary exposure to the gas. An appropriate container should be used to release the gas. The sides of the container should slope outwards to avoid an excessive build-up of heat, which could ignite the formaldehyde. The container should be made of heat-proof material, such as metal or earthenware, and should be sufficiently large to prevent the chemicals from boiling over.
- Liquid formalin 45-60 ml/ cubic meter could be used in the electric evaporator for different rooms inside the hatchery or inside hatchers normal vapours. In addition, some add water to formalin to have dilution at 5% and spray to different objects in a hatchery.
Neutralisation of formaldehyde gas; Formaldehyde gas may be neutralised in 10-15 min using ammonium hydroxide at an amount equal to half the volume of formalin used.
Hazards of fumigation:The human health risks of formaldehyde fumigation are a cause of great concern. Use of formaldehyde is prohibited in some countries. Human exposure should be avoided, and gas masks and protective clothing are essential.
Comparison of Formadehyde and altrantives egg disnfication: (Review)
At the study by W.L.dosS.Cliranco et.al.2018 to evaluate the effect of different disinfection procedures as alternatives to formaldehyde fumigation on eggshell microbial load and quality of eggs; the results showed that, counts of total aerobic mesophilic bacteria and Enterobacteriaceae before and after the disinfection of eggshells of broiler eggs showed in Table 1 below, its can be noted clearly that , the Formalehyde used 13.3 Grams / 1 cubic meter, was have the lowest counts of bacteria after treatmeants compared to other altrantive difinfactants used in their experiments.
Table 1. Counts of total aerobic mesophilic bacteria and Enterobacteriaceae before and after the disinfection of eggshells of eggs from a 42-week-old Cobb broiler breeder flock, using the formaldehyde, ozone, ultraviolet light, hydrogen peroxide, peracetic acid, wet control (water spraying), and dry control (no disinfection) treatments, followed by the respective standard deviations (SD).
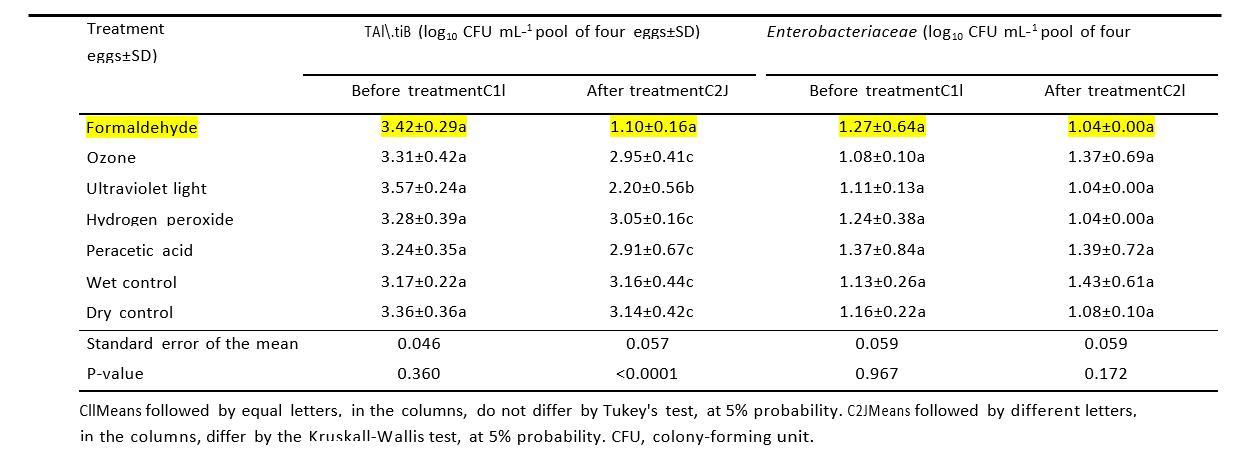
While other study by Melo et.al.2019 used different concentration formaldehyde (5.03 g/m3 /30 min) than, W.L.dosS.Cliranco et.al.2018 concluded that, due to their significant bactericidal effect on the eggshell, and the absence of negative effects on eggshell quality and incubation parameters, UV-C and PAA are indicated as effective alternatives for dis-infecting hatching eggs in old broiler breeder flocks.
Introduce high concentration up to of fumuigation by formalddehyde will affect negatively,the Tracheal Epithelium of Chicken Embryos and Chicks as invistigated by Hayretda and Kolankaya; (2008), they were used a methods of formaldehyde fumigation was applied to 18-day-old embryos and 1-day old chicks only once, at 1 of 2 different concentrations (3×, 42 ml of formalin and 21 g of potassium permanganate per m3 and 4×, 56 ml of formalin and 28 g of potassium permanganate per m3) for 1 of 2 different durations (20 min and 40 min).They conculded that, the tracheal epithelial cells were examined with transmission electron microscopy (TEM). According to TEM, after fumigation cilia in the epithelial cells were shorter and fewer in number, and vacuolisation, swelling of the mitochondria, and spoiling of cristae were observed in the subjects, which varied according to fumigation concentration and duration.
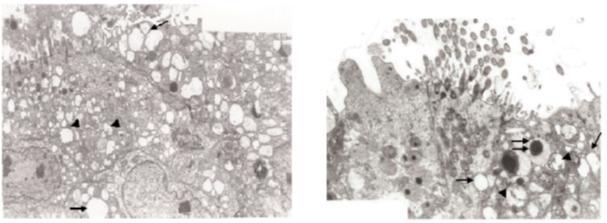
Fig. 1 Fig. 2
Swelling of mitochondria, spoiling of cristae (arrow head), and vacuolisation (arrow) in the tracheal epithelial cells of an 18-day-old embryo exposed to the 4× fumigation for 40 min (Figure 1), Vacuolisation (arrow), mitochondrial defects (arrowhead), and lysosomes (double arrow) in the tracheal epithelial cells of a 1-day-old embryo exposed to 4× fumigation for 40 min (Figure 2).
Finally, the aouthers summarized that , according to TEM investigation, shortening and loss of cilia in the epithelial cells, vacuolisation, swelling of the mitochondria, and spoiling of their cristae were present in both 18-day-old embryos and 1-day-old chicks. Extending the fumigation period caused increases in these effects. An important factor in the effect of formaldehyde on the tracheal mucosa is the dissolution of the gas in secretions. Formaldehyde dissolved in mucous secretions causes a pH shift toward acidity and these changes in pH cause damage to the membrane structure and ciliary activity. This study also shows that liquid and ion alteration may cause mitochondrial damage and vacuolisation in epithelial cells.
Badran A.M.M et.al 2018 studied to investigate the effect of some different chemical egg disinfectants (Formaldehyde, Hydrogen peroxide H2O2 and TH4) against bacteria and to study its effect on embryonic mortality, hatchability and some blood biochemicals parameters. (formaldehyde, obtained from 60 mL formalin, 30 mL water and 48 g potassium permanganate at a temperature of 37оС, air humidity 80% and exposure time 30 minutes)concering on effect of disinfecatants on hatchability pramaters was postive compared to H2O2 and TH4 as showen in tables 2, 3 below:
Table 2 : Effects of the some disinfectants on embryonic mortalities.
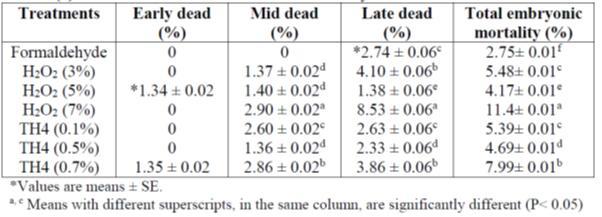
Table 3: Effects of the some disinfectants on the fertility and hatchability traits.
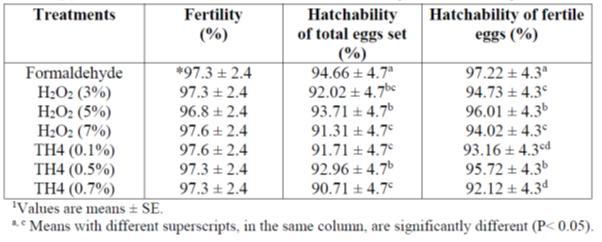
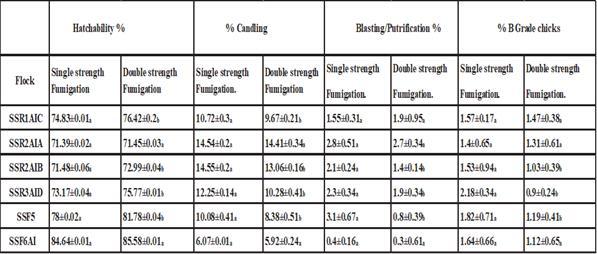
Although the Formaldehyde had the best hatchability traits % could be notice from above tables, but the blood praramters was less compared to H2O2 and TH4. Adnan. J. et.al (2019) studied the use of double strength fumigation with formaldehyde gas on broiler breeder’s eggs hatchability, candling, blasting/putrification, dead in shell, quality of chicks and later life mortality. The fumigation of both groups was performed by automatic fumigating system provided by chick master .The eggs from group A were fumigated with 20gram KMNO4 40 ml (40% aqueous) formalin along 40 ml water for 100ft3 for20 minutes according to formula L*W*H*20/100ft3 (for single strength), while eggs from group B were fumigated L*W*H*40/100ft3 (double strength) for 20 minutes. After fumigation both groups were immediately shifted to incubation.( 1 m3 = 35.3146667 ft3).
The results showed in Table (4), the authers concluded that, The fumigation of both groups was performed through automatic fumigation system provided by chick master. The hatchability and candling of flocks SSR1AIC, SSR2AIB, SSR3AID and SSF5 were significantly <0.05 better for double strength fumigation compared to single strength fumigation. The dead in shell were insignificant for the treatment. The putrification/blasting and low quality chicks were significantly <0.05 better for double strength fumigation for SSR2AIB, SSR3AID and SSF5, while remaining flocks were insignificant regarding quality of chicks and putrification due to fumigation strength. The chicks each (n = 30,000) were shifted to poultry houses from both groups to know the effects of fumigation strength on later life of chicks. The weight gain, feed intake and FCR were insignificant for both groups while mortality was significantly better for double strength fumigation compare to single strength fumigation. In short double strength fumigation is safe can be used to improve the hatchery parameters and later life of chicks.
Table 4: Effect of Fumigation strength on Hatchability, Candling and Blasting/Putrification and B grade chicks a-b denote difference in rows (p<0.05)
Russell (1976) indicated that formaldehyde acts on proteins and also on nucleic acids. It is feasible that formaldehyde gas diffused into the egg at an early stage of embryonic development will alkylate the nitrogen atoms of purine and pirimidine bases in DNA and RNA thus inhibit-ing their function. This, in turn, it can block embryonic development at an early stage, even prior to incubation. Fumigation near the time of hatching can also result in embryo mortality. The reason is that formaldehyde a hazardous gas may damage airways and lungs, when inhaled.
Cadirci.S (2009) summarized in his review that, for disinfection against Salmonella species, nest clean eggs should be fumigated prior to incubation at room temperature (25°C) and ambient humidity for at least 20 minutes with a minimum concentration of 600 mg formaldehyde gas per m3 (i.e. 10 g paraformaldehyde or 45 ml of 40% formalin and 30 g KMnO4). Fumigation under these conditions kills 99.8% of microorganisms on the shell surface and is not associated with increased embryonic mortality. Fumigation should not be performed during the first 9 days of incubation. Fumigation at pipping may damage the respiratory system of chicks and have adverse effects on the health and production performance. In addition, formaldehyde is also hazardous to human health.
Paula Johnson, 2018 concluded that, The use of formaldehyde has proven to be extremely effective for an array of uses including clothing production, lumber manufacture, cosmetic manufacture, general disinfectant use, sanitizing poultry eggs, and even use in hatching cabinets during the hatching process. This molecule is very effective as a disinfectant in the poultry industry in reducing the load of microorganisms. Its role in the reduction of pathogenic microorganism’s aids in decreasing the potential for pathogenic or opportunistic pathogenic microorganisms reaching the embryo resulting in decreased hatchability and embryonic death, or neonatal infections resulting in early mortalities after placement. In the absence of alternatives that are cost- and labor-effective, formaldehyde will continue .
Summary and Recomindation:
Currently, Formaldehyde is the most common powerful disinfectant of the poultry industry, and disinfect of hatching eggs in hatcheries to prevent pathogens transmissions. it has a wide effective to kill most of microorganisms and pathogens and low cost. there are several methods to generate formaldehyde, the commonly used is the addition of formalin to potassium permanganate (KMnO4) in 2:1 ratio (v/w), could be replaced recently by Formaldehyde volatilisation of the polymer paraformaldehyde under controlled temperature system.
There are some factors influences of efficiency of formaldehyde fumigation to maximize germicidal activity from formaldehyde i.e temperature, humidity, time of exposure, the concentration of formaldehyde, circulation of gas, the amount of organic matter on the shell surface and method used of generating formaldehyde vapours.
Events of formaldehyde fumigation use at high concentration or long exposer time cause early embryonic deaths before incubation and in late-stage of embryos will be affected negatively, the Tracheal Epithelium of chicken embryos and chicks, cilia in the epithelial cells were shorter and fewer in number, and vacuolisation, swelling of the mitochondria, and spoiling of cristae were observed in the subjects, which varied according to fumigation concentration and duration.
For the maximum benefits of formaldehyde to kill 99.8 % of microorganisms, pathogens, and neglected negative effect on the eggshell cuticle and improve hatchability and produce healthy chicks performed well at the farm with low mortality % and better FCR, should consider the proper way discussed in this papers. Precautions of using formaldehyde should take in proper practice to avoid any hazard to human health.
Hatcheries should have an especial fumigation room or chamber to prevent the hazard of formaldehyde on workers and hatchery production. such the room must provide some requirements and conditions as follow :
- The chamber must be well insulated.
- Provide good ventilation.
- Enough spaces between trolleys for good air circulation.
- Provide strong exhaust fans to the atmosphere after Neutralisation when needed.
- Temperature and humidity control.
- Provide a source of heating Paraformaldehyde, or/ and proper metal tank when use formalin- potassium permanganate method.
- The chamber build material not able to absorb or keep residual formaldehyde gas.
- Prefer to provide automation control to the fumigation chamber.
Other Recommendations:
After hatching eggs fumigated, prefer to keep eggs inside the chamber a few hours to be sure there is no residual gas remain on egg shell (Smell), for not affect negatively, the very early stage of embryos.
EmTech Fumigation Room Technology (EFRT):
EmTech hatchery system provides the most advanced technology to fumigate hatching eggs use formaldehyde in hatcheries, EmTech implemented the scientific, technical, recommendation and best practice mentioned in this article, to their design of Fumigation room.
The fumigation room built with different size (W x Lx H) to be design related to hatchery production capacity and customers needs, full insulation room, uses of antifire certificated PIR, and international standard antimicroorganisms food safety panels, and stainless stells doors have magnetic locks (See Photos1,2)
Photo:1 Photo:2


Those doors and panels completely insulated prevent leaks of formaldehyde gas outside the room, for healthy of staff and keep hatchery environment safe of formaldehyde as (OSHA) recommendations. In addition, the material used not able to absorb formaldehyde gas, or keep any gas residual after fumage eggs operation for not affect negatively very early embryonic development inside the modern incubators.
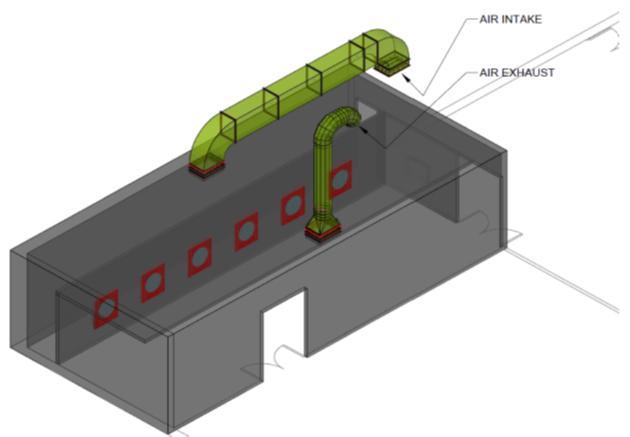
The fumigation room has a ventilation system that contains inlet air damper and exhaust gas fan duct and 6 fans at wall side for formaldehyde gas distributed evenly around all eggs inside the fumigation room. (See photos 3, 4 and Digram1) And airpresuers control.
Photo 3 Photo 4


Diagram 1
EmTech fumigation room has front screen control to automatically control all fumigation process, temperature, humidity, heaters and timer, provided also with pan heater for use of paraformaldehyde and pan/s activates a second electrical output for heater pan(s) containing a neutralisation (See Diagram 2). Moreover, the fumigation room control can be remotely operated through EmTch hatchery system monitoring and ability to save data for every fumigation room operation to assist the hatchery management team for further investigation and secure standard fumigation room process control.
Diagram:2

The control of fumigation room has manual/ automatic check for every option before fumigation run, to ensure that all room functions are run correctly. Moreover, if only 1 option fails the all system will not run for the safety of both human and environment outside and inside the hatchery.
There are many other options for fumigation room users, including user login, alarms of low and high values of temperature, humidity, fans speeds and air pressures, emergency stop as described in EmTech FUMIGATION ROOM USER MANUAL.
Mohamed El-Ashram – EmTech Global Hatchery Support Director
Mobiles +44 7795538884 or +201033383880
www.EmTech-Systems.com
Lopen Business Park, Mill Lane, Lopen,
South Petherton, Somerset, TA135JS, UK
Referances:
Adnan JAbbAr, Adnan YousAf, Abdul HAmeed, AmJAd riaz, Yasir AllAH ditta., 2019: Influnces of Fumigation Strength on Hatchery Parameters and Later Life of Chicks. J Holistic Vet Sci Ani Care 1(1): 101.
Badran A.M.M; A. M. R. Osman and D. M.M. Yassein., 2018: COMPARATIVE STUDY OF THE EFFECT OF SOME DISINFECTANTS ON EMBRYONIC MORTALITY, HATCHABILITY, AND SOME BLOOD COMPONENTS. Egypt. Poult. Sci. Vol. (38)(IV): (1069-1081).
Cadirci S. (2009). Disinfection of hatching eggs by formaldehyde fumigation – a review. Arch Geflügelk 73. 2 S: 116-23.
Hayretda. S and Kolankaya D.,2008: Investigation of the Effects of Pre-Incubation Formaldehyde Fumigation on the Tracheal Epithelium of Chicken Embryos and Chicks. Turk. J. Vet. Anim. Sci. 32(4): 263-267
Melo E. F., W. L. S. Cl´ımaco, M. V. Triginelli, D. P. Vaz, M. R. de Souza, N. C. Bai˜ao, M. A. Pompeu, and L. J. C. Lara., 2019: An evaluation of alternative methods for sanitizing hatching eggs. Poult. Sci. 98:2466–2473.
RUSSELL, A.D., 1976: Inactivation of non-sporing bacteria by gases. Soc. Appl. Bacteriol. 5, 61-68.
Paula Johnson.,2018: Evaluation of the Effects of Formaldehyde on Growth Parameters of Broiler Chicks. Master theses; University of Arkansas, Fayetteville. http://scholarworks.uark.edu/etd.
- L. S. Cl´ımaco ., Melo E. F., M. V. Triginelli, D. P. Vaz, M. R. de Souza, N. C. Bai˜ao, M. A. Pompeu, and L. J. C. Lara., Mariana M. S., Maria F. V., Letícia C. F., Nelson C. B., Felipe M. de Souzaand.,2018: Eggshell microbiology and quality of hatching eggs subjected to different sanitizing procedures. Pesq. agropec. bras., Brasília, v.53, n.10, p.1177-1183.
https://pubchem.ncbi.nlm.nih.gov/compound/Formaldehyde
https://www.chemicalsafetyfacts.org/formaldehyde
https://www.epa.gov/indoor-air-quality-iaq
https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_id=10075&p_table=STANDARDS


